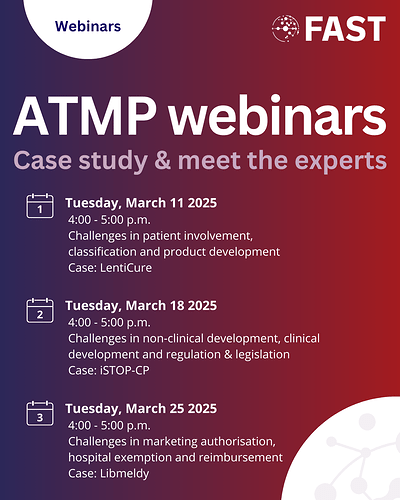
To support innovators, FAST is hosting a case-based webinar series on ATMPs, featuring expert insights and practical guidance. Each session starts with a real-world case study followed by an interactive Q&A led by experts on selected topics from the FAST ATMP guidebook. In this webinar we will be joined by iSTOP-CP and will cover regulation, non-clinical, and clinical development.
Have questions already? Submit them in advance to our expert panel by posting them here!
For more information visit the website and sign up here.
Tuesday, March 18: Webinar about Challenges in regulation & legislation, non-clinical development and clinical development of ATMPs
Case study: iSTOP-CP: intranasal stem cells to treat perinatal brain injury and combat cerebral palsy by Cora Nijboer @chanijboer and Manon Benders @mjnlb
You can choose one of these 3 ‘meet-the-expert’ sessions:
- Regulation and legislation by Ineke Jonker-Hoogerkamp (Eagle Pharma Consult)
- Clinical development by Joop van Gerven (CCMO CHDR)
- Non-clinical development by Trudy Straetemans (UMC Utrecht) @Trudy & Jolanda Liefhebber @J.Liefhebber
Feel free to ask your follow-up questions in the comments on the Forum!
Update: thank you for joining the webinar! The case studies have been recorded, here is the link to watch it back.
 Case recordings ATMP webinar 2: iSTOP-CP (UMC Utrecht)
Case recordings ATMP webinar 2: iSTOP-CP (UMC Utrecht)
1 Like