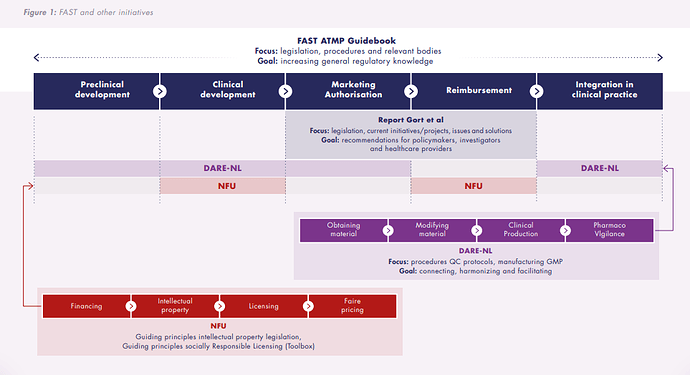
FAST has collaborated with experts to create a guidebook for developing Advanced Therapy Medicinal Products (ATMPs) in the Netherlands. Tailored to both academic and commercial developers, the guidebook delivers essential guidance on European and Dutch regulations and provides a roadmap to navigate the complex ATMP development process.
A guide to the ATMP landscape
The guidebook is the result of close collaboration with key partners, including the Dutch Medicines Evaluation Board (CBG), Hollandbio, DARE-NL, VIG, and input from over 25 experts. Building on the foundation of the VIG’s earlier guidebook, this updated and expanded edition integrates the latest insights, regulations, EMA processes, and advances in clinical research. With its practical tools and guidance, the handbook equipes developers to handle complex procedures such as marketing authorization and reimbursement applications more effectively.